Gene Expression and Epigenetics: How Diet and Environment Shape Your Health
It is not genes that matter but the way they express themselves.
Milos Pokimica
Written By: Milos Pokimica
Medically Reviewed by: Dr. Xiùying Wáng, M.D.
Updated January 5, 2026Key Takeaways:
-Gene expression and epigenetics are dynamic and responsive processes that regulate how genes are turned on or off in response to various stimuli.
-Diet and environment are two major factors that can influence gene expression and epigenetics, by providing nutrients, chemicals, or signals that can modify the DNA or the proteins that interact with it.
– Changes in gene expression can have significant effects on health and disease prevention, by altering the cellular functions, metabolism, inflammation, immunity, and aging of the body.
Introduction.
Most people are familiar to some extent with the science behind genetics. Genetics helps us understand how evolution works, and how we inherit traits from our parents and helps us in medicine, or anthropology by understanding how our bodies evolved from hominins to anatomically modern humans.
But what about epigenetics? Are you familiar with this term? Do you know that genes are not set in stone? Do you know how we can change our destiny by influencing our genes? It is a new and emerging scientific field of epigenetics.
Classical genetics.
But how did we get here? How did we discover the secrets of DNA and the mechanisms that regulate it?
It all started with a monk named Gregor Mendel, who lived in the 19th century. He was curious about how plants inherited traits like flower color and seed shape. He did experiments with pea plants, crossing them and counting the offspring. He noticed that some traits followed simple patterns of inheritance, while others seemed to blend or disappear. He came up with some rules that explained his observations, which we now call Mendel’s laws of inheritance. He published his work in 1865, but nobody cared. He died without knowing that he was the father of the most important field in science called genetics.
Fast forward to the early 20th century. Some scientists rediscovered Mendel’s work and realized that it was genius. They also discovered that chromosomes were made of DNA and proteins and that they carried the units of inheritance, which they called genes. They figured out how genes were arranged on chromosomes, how they could be exchanged during meiosis (cell division), and how they could be mutated by radiation or chemicals. They also learned how to map genes on chromosomes using linkage analysis and recombination frequencies. This was the era of classical genetics.
But there was a problem. Classical genetics could not explain everything. For example, how did genes control traits? How did genes interact with the environment or how much of it has any effect on our genome? How did genes change over time and across generations? These questions led to the creation of a new discipline called molecular biology.
Central dogma.
In 1953 James Watson and Francis Crick solved the structure of DNA. It was a double helix made of four nucleotides (A, T, C, G) that paired with each other in a specific way (A with T, C with G). They realized that this structure explained how DNA could store information (the sequence of nucleotides), copy itself (by separating the strands and using them as templates), and express itself (by being transcribed into RNA and translated into proteins). This work became the ”central dogma of molecular biology”. A theory states that genetic information flows only in one direction, from DNA, to RNA, to protein, or RNA directly to protein. It was first stated by Francis Crick in 1957 and published in 1958.
But there was another problem. Molecular biology could not explain everything either.
For example, how did cells know when and where to turn genes on and off? During development, how did cells divide into distinct types because every single one has the same DNA? How did cells remember their identity and history which is important for cancer research? How did cells respond to signals from other cells or from the environment?
Birth of epigenetics.
These questions led to the birth of epigenetics, which aimed to reveal the mechanisms that regulate gene expression without changing the DNA sequence.
The term epigenetics were coined by Conrad Waddington back in 1942, but it took decades for it to gain scientific acceptance.
Epigenetics is based on the idea that there are chemical modifications on DNA or on histones (proteins that wrap around DNA) that can affect how genes are accessed and used by the cell machinery. These modifications can be added or removed by enzymes, depending on various factors such as cell type, developmental stage, environmental cues, stress, diet, and so on (Peixoto et al., 2020). These changes can also be passed down to daughter cells during cell division, or even to children during reproduction.
This means that epigenetics can influence traits that are not encoded by DNA alone, such as behavior, disease susceptibility, or aging.
Epigenetics is one of the hottest topics as same as genetic once was even more. The problem is that most people did not hear about it so the old belief remains that genes are everything. We need to popularise the new research to the broader public that still believes that genes are set in stone and that there is nothing you can do about it. Epigenetics challenges some of the assumptions and dogmas of genetics and molecular biology. It opens new possibilities for understanding life at a deeper level. It also opens up new avenues for enhancing medicine and health by modifying gene expression.
It is not genes that matter but the way they express themselves.
What is Gene Expression?
Before we dive more scientifically into epigenetics, let’s talk about gene expression.
Gene expression refers to how often or when proteins are created from the instructions within your genes (What Is Epigenetics? | CDC, 2022b).
Proteins are the building blocks. They perform many functions, such as building tissues, fighting infections, and regulating hormones (Pelusa et al., 2022).
Your genes carry the information for producing proteins, but they do not produce proteins themselves. They require the assistance of other molecules, like RNA and enzymes, to read the code and produce the proteins. This process is called transcription (Blackwell et al., 2006).
Transcription is not always on. It can be on or off depending on different factors, such as signals from other cells, hormones, or nutrients. This is how our body adapts to different situations.
For example, when you’re hungry, your body turns on genes that make enzymes break down food and release energy. When you’re full, your body turns off those genes and turns on genes that store excess energy as fat.
Why Does Epigenetics Matter?
Epi means above or ‘on the top of’ in Greek. It is not your genes that matter but what they express.
All your cells have the same genes but look and act differently because of epigenetic changes. In other words, it is an additional layer of information that controls gene regulation (Hamilton et al., 2011).
Epigenetic modifications can inhibit or activate gene expression. A combination of these modifications plays an important role in “imprinting,” a type of “mark” that determines whether a gene will be expressed or not.
DNA is formed by combinations of nucleotides. These are the famous letters most people know about, adenine (A), thiamine (T), guanine (G), and cytosine (C).
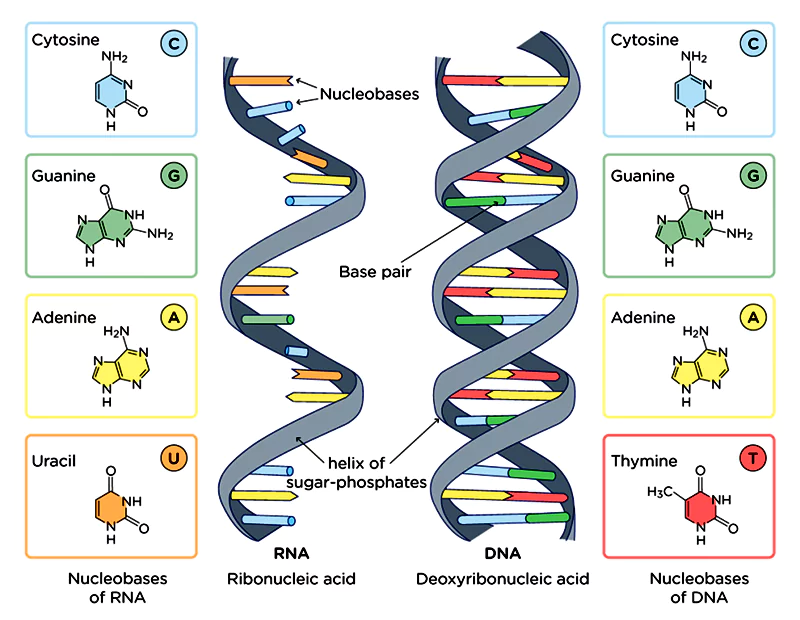
Cytosine, for example, can be changed by adding a methyl group (CH3).
This process is known as methylation, and it alters the expression of the gene whose sequence has been altered. DNA methylation typically leads to the silencing of the gene. In the wrong circumstances, this could be very bad if the gene regulates the immune or some other important function.
Epigenetic changes can happen even before you’re born, during your development, and throughout your life. Some epigenetic changes are normal and necessary for your growth and function.
Other epigenetic changes are influenced by your behaviors and environment.
For example, what you eat can affect how your genes are methylated or how your histones are modified.
Your physical activity can affect how much non-coding RNA is produced or how it interacts with coding RNA.
These epigenetic changes are part of evolution and have their purpose but also in some scenarios can have positive or negative effects on your health.
Some epigenetic changes, for example, can improve our immune system by activating genes that fight inflammation or cancer.
Other epigenetic changes can increase your risk of diseases by turning off genes that regulate metabolism or the immune system (Fanucchi et al, 2021); (Surace et al., 2019).
This is a driving force of adaptation and evolution. If you want proof of evolution and how it works in real-time and how organisms evolve this is it. This is a reason why epigenetic changes can also be inherited from one generation to another. For example, if your grandparents were exposed to toxins or mutagens or stressors and if that exposure resulted in epigenetic changes in their DNA or sperm cells or egg cells, those changes could be then passed on to you and affect your gene expression (Denhardt et al., 2018).
Having a polluted environment and toxins everywhere will not just affect your DNA but also the DNA of your children (de Magalhães-Barbosa et al., 2022).
Epigenetic Changes and Their Effects on Health.
The table below illustrates the main factors that influence gene expression and epigenetic changes. There are more factors but what you can take home from this table is the fact that in every row the factors that influence the risk are diet, stress, and exposure to toxins.
| Epigenetic Changes | Effects on Health | Sources |
| DNA methylation | A process that adds methyl groups to DNA bases, affecting gene expression. Environmental variables such as food, stress, and toxicity exposure can all alter DNA methylation. DNA methylation can affect various aspects of health, such as cancer risk, immune function, and aging. | Cavalli et al., 2019 |
| Histone modifications | A process that alters the structure of histones, the proteins that wrap around DNA. Histone modifications can affect how tightly or loosely DNA is packaged, affecting gene expression. Histone modifications can be influenced by environmental factors such as diet, stress, and exposure to toxins. Histone modifications can affect various aspects of health, such as cancer risk, immune function, and aging. | Cavalli et al., 2019 |
| Non-coding RNA | RNA that does not code for proteins. Environmental factors like diet, stress, and toxin exposure can all affect non-coding RNA. Non-coding RNA can have an impact on many areas of health, including cancer risk, immunological function, and aging. | Cavalli et al., 2019 |
| Infections | Germs can change your epigenetics to weaken your immune system. This helps the germ survive. Example: Mycobacterium tuberculosis causes tuberculosis. Tuberculosis was and still could be a fatal disease because it can alter the DNA methylation of immune cells, thus making the immune system less effective at fighting the infection. | What Is Epigenetics? | CDC, 2022b |
Examples of Epigenetic Changes and Their Effects on Cancer Risk.
Cancer is a complex disease that involves thousands of different mutations that accumulate and involves both changes in the genome and the epigenome (Brena et al., 2007), (Shen et al., 2013). You can have a genetic predisposition that you are enheartened from your parents but that is just one part of the entire picture.
That genetic predisposition for cancer actually turns itself on depending on gene expression and gene expression mostly depends on a diet (Hullar et al., 2014), and environment (Abdul et al., 2017).
Epigenetic changes can turn on or off genes that are involved in cell growth, cell death, or immune response. These changes can then affect your overall risk of developing cancer or responding to cancer treatment. This is why we have an epidemic of cancer where one in four people in Western societies with a standard American diet will die from it. It is not bad genes that are a cause, but gene expression.
Here are some examples of epigenetic changes and their effects on cancer risk:
- DNA methylation blocks the proteins that read the gene from accessing it, essentially turning the gene off. DNA methylation can turn off genes that suppress tumors or repair DNA damage basically deactivating the immune system. For example, having a mutation in the BRCA1 gene that prevents it from working properly makes you more likely to get breast and some other types of cancers as well. However, if this gene is also methylated, then you are going to die most likely because your immune system is turned off. Methylation can further increase your cancer risk and make your cancer more aggressive (Catteau et al., 2002), (Prajzendanc et al., 2020).
- Histone modification is when chemical groups are added or removed from histones, which are proteins that wrap around DNA to form a structure called chromatin. Depending on the type and location of the chemical groups, histone modification can make chromatin more tightly or loosely packed, affecting how much DNA is exposed or hidden from the proteins that read it. Histone modification can affect genes that regulate the cell cycle, apoptosis, or angiogenesis. For example, having a mutation in the p53 gene that prevents it from working properly makes you more likely to get various cancers. However, if this gene is also modified by histones, it can further increase your cancer risk or make your cancer more resistant to treatment (Yue et al., 2017).
- Non-coding RNA: This is when molecules called non-coding RNA to attach to coding RNA, which is used to make proteins. Non-coding RNA can help break down coding RNA or recruit molecules that modify histones, affecting gene expression. Non-coding RNA can affect genes that control cell differentiation, invasion, or metastasis. For example, having a mutation in the KRAS gene that prevents it from working properly makes you more likely to get colorectal cancer. However, if this gene is also regulated by non-coding RNA, it can further increase your cancer risk or make your cancer more difficult to treat (Saliani et al., 2022).
Epigenetic Changes and Their Effects Depending on Diet and Nutrition
| Dietary components | Epigenetic Changes | Sources |
| Antioxidants | Antioxidants are molecules that can neutralize free radicals, which can damage DNA and histones. Antioxidants can modulate epigenetic changes by affecting DNA methylation and histone modifications. | Beetch et al., 2020 |
| Folate | Folate is a B vitamin that is involved in the synthesis of DNA and RNA. Folate can affect epigenetic changes by providing methyl groups for DNA methylation. Folate deficiency can impair DNA methylation and increase the risk of various diseases, such as cancer, neural tube defects, and cognitive impairment. | Crider et al., 2012 |
| Calorie restriction | Calorie restriction is a dietary intervention that reduces the intake of calories without causing malnutrition by the downregulation of various factors, such as inflammation, oxidative stress, and basal metabolic rate. Calorie restriction can affect epigenetic changes by altering the expression and activity of enzymes involved in DNA methylation and histone modifications. | Gensous et al., 2019 |
| Fiber | Fiber is a type of carbohydrate that is not digested by human enzymes but can be fermented by gut bacteria. Fiber can affect epigenetic changes by influencing the composition and function of the gut microbiota, which can produce metabolites that modulate DNA methylation and histone modifications. | Choi et al., 2010 |
| Probiotics | Probiotics can affect epigenetic changes by influencing the composition and function of the gut microbiota, which can produce metabolites that modulate DNA methylation and histone modifications. Probiotics can also modulate the expression of genes involved in inflammation, immunity, and metabolism. | (Borzabadi et al., 2018), (Ye et al., 2017) |
| Prebiotics | Prebiotics are non-digestible carbohydrates that selectively stimulate the growth and/or activity of beneficial gut bacteria. Prebiotics can affect epigenetic changes by influencing the composition and function of the gut microbiota, which can produce metabolites that modulate DNA methylation and histone modifications. Prebiotics can also modulate the expression of genes involved in inflammation, immunity, and metabolism. | Ye et al., 2017 |
Antioxidants’ effect on gene expression.
Here are some examples this is not an entire list of all effects, just an example:
- Antioxidants can prevent or reverse DNA methylation. DNA methylation can happen when you are exposed to free radicals or toxins. Antioxidants can block this process or remove the methyl group, restoring the gene function.
- Antioxidants can modulate histone modifications. Antioxidants can influence the enzymes that make these modifications, changing the chromatin structure.
- Antioxidants can regulate non-coding RNA. Antioxidants can affect the production or activity of non-coding RNA, altering gene regulation.
- Antioxidants can prevent or repair DNA damage and restore normal gene expression, preventing or slowing down cancer development.
In the table below are selected antioxidants and their effects on gene expression. This is not a complete list just some examples that have been researched by the scientist. There are thousands of different phytochemicals and you should or would not be able to research them all. You should strive to increase the overall ORAC value of your diet through nutrition and not go with the route of individual supplemental antioxidants. Phytochemicals work synergistically as a complex of chemicals from whole food sources where 2 plus 2 equals 15. In the table, I have listed some antioxidants just as an example.
| Antioxidant | Effect on epigenetics | Source |
| Curcumin | Curcumin is an anti-inflammatory, antioxidant, and anticancer polyphenol produced from turmeric. Curcumin can inhibit DNA methyltransferases (DNMTs) and histone deacetylases (HDACs), causing tumor suppressor genes to reactivate and oncogenes to be suppressed. Curcumin can also change chromatin structure and gene expression by inducing histone acetylation and methylation. Curcumin can modulate microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), both of which target genes involved in inflammation, apoptosis, cell cycle, invasion, and metastasis. | Bhattacharjee et al., 2020 |
| Resveratrol | Resveratrol is a natural polyphenol found in grapes, red wine, berries, and peanuts that has antioxidant, anti-inflammatory, and anticancer effects. Resveratrol can inhibit DNMTs and HDACs, resulting in the demethylation and reactivation of tumor suppressor genes and the downregulation of oncogenes. Resveratrol can also induce histone acetylation and methylation, affecting the chromatin structure and gene expression. Resveratrol can regulate miRNAs and lncRNAs that target genes involved in oxidative stress, inflammation, apoptosis, autophagy, senescence, angiogenesis, and metastasis. | Griñán-Ferré et al., 2020 |
| Apigenin | Apigenin is a natural flavonoid derived from chamomile flowers, oranges, parsley, celery, and other natural sources that has antioxidant, anti-inflammatory, and anticancer properties. Apigenin can inhibit DNMTs and HDACs, leading to the demethylation and reactivation of tumor suppressor genes and the downregulation of oncogenes. Apigenin can also induce histone acetylation and methylation, altering the chromatin structure and gene expression. Apigenin can regulate miRNAs that target genes involved in the cell cycle, apoptosis, invasion, metastasis, angiogenesis, and stemness. | Bhattacharjee et al., 2020 |
| Sulforaphane | Sulforaphane is a natural isothiocyanate derived from cruciferous vegetables such as broccoli, cabbage, and kale that has antioxidant, anti-inflammatory, and anticancer properties. Sulforaphane can inhibit DNMTs and HDACs, leading to the demethylation and reactivation of tumor suppressor genes and the downregulation of oncogenes. Sulforaphane can also induce histone acetylation and methylation, altering the chromatin structure and gene expression. Sulforaphane can regulate miRNAs and lncRNAs that target genes involved in inflammation, apoptosis, cell cycle, invasion, and metastasis. | Bhattacharjee et al., 2020 |
| Ursolic acid | Ursolic acid is a natural pentacyclic triterpenoid found in various fruits, herbs, and spices that has antioxidant, anti-inflammatory, and anticancer effects. Ursolic acid can inhibit DNMTs and HDACs, resulting in the demethylation and reactivation of tumor suppressor genes and the downregulation of oncogenes. Ursolic acid can also induce histone acetylation and methylation, affecting the chromatin structure and gene expression. Ursolic acid can regulate miRNAs that target genes involved in the cell cycle, apoptosis, invasion, metastasis, angiogenesis, and stemness. | Bhattacharjee et al., 2020 |
| Allicin | Allicin is a natural sulfur compound derived from garlic that has antimicrobial, antioxidant, anti-inflammatory, and anticancer effects. Allicin can inhibit DNA gyrase activity in bacteria, leading to the inhibition of DNA replication and transcription. Allicin can also oxidize cysteine residues in proteins, affecting their structure and function. Allicin can regulate miRNAs that target genes involved in the cell cycle, apoptosis, invasion, metastasis, angiogenesis, and stemness. | Chhabria et al., 2015 |
There are many more antioxidants that can affect gene expression. If you want to learn more, you can search for correlated articles.
You might also be wondering where you can get antioxidants from. The good news is that they are mostly found in plants and that there are plants that are very rich in antioxidant content. Learn your ORAC values.
Folate effects on gene expression.
Folate is a B vitamin that is involved in the synthesis of DNA and RNA. You need folate for your cells to grow and divide properly. You can get folate from foods like leafy greens, beans, nuts, eggs, and fortified cereals. The problem is that this is one of the most prevailing deficiencies in the standard American diet. People eating a whole-food plant-based diet usually are not deficient in folate and do not need an additional folic acid supplement.
Here are some examples:
- Folate provides methyl groups (one carbon group) for DNA methylation (Crider et al., 2012). Folate is one of the main sources of methyl groups for this process. Folate deficiency can impair DNA methylation and cause abnormal gene expression.
- Folate affects neural tube closure. The neural tube is the structure that forms the brain and spinal cord in the embryo. The neural tube needs to close properly for normal development. Folate is essential for this process because it affects the expression of genes involved in neural tube closure (Saitsu et al., 2017). Folate deficiency can prevent neural tube closure and cause birth defects such as spina bifida.
- Folate is important for cognitive function because it affects the expression of genes involved in brain development and function. Folate deficiency can impair cognitive function and increase the risk of dementia.
These are some of the effects of folate on epigenetics and why they are important for your health and development. You might be wondering how much folate you need and where you can get it from. Well, here are some tips:
- The RDA for folate is 400 micrograms for adults and 600 mcg for pregnant women.
- You can get folate from foods such as leafy greens, beans, nuts, eggs, and fortified cereals. You can also take a supplement if you have a deficiency or a higher need for folate.
- You should avoid taking too much folate because it can mask a vitamin B12 deficiency or interfere with some medications.
- Also folic acid is not the same as folate. We need folate but supplements are made of folic acid. Our liver unlike the liver of rats in the animal model is unable to convert more than 400mg of folic acid into folate in one day. That is why most supplements never exceed more than 400mg of folic acid.
Calorie restriction effects on gene expression.
Calorie restriction is when you reduce your calorie intake without causing malnutrition. Calorie restriction can affect how much non-coding RNA is produced or how it interacts with coding RNA (Abraham et all., 2017). Calorie restriction can also regulate the circadian rhythm of gene expression in different organs and tissues (Patel et al., 2016).
These effects of calorie restriction on epigenetics can have various benefits for your health and aging. For example:
- Calorie restriction can slow down aging by modulating various pathways, such as inflammation (Gabandé-Rodríguez et al., 2019), oxidative stress, metabolism, and autophagy (Bagherniya et al., 2018).
- Calorie restriction can also extend lifespan by increasing the expression of genes that protect against cellular damage and death (Komatsu T et al., 2019).
In our normal evolution, we were forced into calorie restrictions in our normal environment for all of our hominin ancestors due to scarcity. Our body’s response to restriction is to repair itself by destroying bad or mutated or precancerous cells first for energy and by slowing down metabolism. When you slow down your metabolism you burn less energy, have lower oxidative stress and you live longer. And calorie restriction also has effects on gene expression. Our body is used to havening it and expects it as a normal part of life. Lacking autophagy directly leads to cancer risk. A whole-food plant-based diet is naturally more inclined to induce calorie restriction by providing fewer calories than a Standard American Diet (SAD) while still meeting nutritional needs (Greger, 2020). SAD on another hand causes calorie excess due to oil and sugar and highly palatable foods, which can tan impair gene expression and increase disease risk.
Fiber effects on gene expression.
Fiber is a type of carbohydrate that is not directly digested by our enzymes but ends up in the colon where it gets fermented by gut bacteria. Fiber can help you regulate your digestion, lower your cholesterol, and prevent constipation.
Bacteria that ferment fiber are symbiotic and good for our immune system and body, unlike non-probiotic bacteria that putrefies meat. This meat-eating bacterium putrefies the flesh you have eaten for hours in the colon causing inflammation. Meat is meat and yours is tasty too.
You can get fiber from foods like fruits, vegetables, grains, beans, and nuts.
Here are some examples:
- Fiber can affect the composition and function of the gut microbiota by providing probiotic bacteria with food and stimulating their growth and activity against meat-eating non-probiotic bacteria (Makki et al., 2018).
- Fiber affects the metabolites produced by the gut microbiota. The metabolites are the substances that are produced or consumed by the gut microbiota. They can enter your bloodstream and affect your organs and tissues. Fiber can affect the type and amount of these metabolites produced by the gut microbiota by stimulating probiotic bacteria and downregulating metabolic processes of non-probiotic meat-eating bacteria in the colon (Makki et al., 2018).
- These metabolites can then affect epigenetic regulation by modulating the availability or activity of chemical donors or enzymes that control DNA methylation and histone modifications. These epigenetic changes can alter the expression of genes involved in inflammation, immunity, and metabolism.
- Fiber can protect against obesity and diabetes by modulating the expression of genes involved in glucose homeostasis, lipid metabolism, and energy expenditure.
- Fiber can improve immune function. Chronic inflammation can be a consequence of an imbalance of the gut microbiota or impaired barrier function of the gut. Fiber can improve immune function and prevent infections by modulating the expression of genes involved in inflammation, immunity, and barrier function and by downregulating the activity and number of non-probiotic bacteria in the microbiota colony. Fiber can also stimulate the production of antibodies and cytokines that help fight off germs.
The recommended dietary intake (RDI) for fiber is 25 grams per day for women and 38 grams per day for men. This is just RDA for SAD. In an anthropological sense, our hominin ancestors have consumed much more than that. One rule with fiber is that more is usually better. The problem is that we do not want bloating, gas, and constantly increased bowel movements. We also don’t like the funky texture of the fiber with no taste so we prefer not to eat it.
In the video below Oded Rechavi, Ph.D., professor of neurobiology at Tel Aviv University and expert in how genes are inherited, how experiences shape genes, and remarkably, how some memories of experiences can be passed via genes to offspring. He discusses his research challenging long-held tenets of genetic inheritance and the relevance of those findings to understanding key biological and psychological processes including metabolism, stress, and trauma. He describes the history of the scientific exploration of the “heritability of acquired traits” and how epigenetics and RNA biology can account for some of the passage of certain experience-based memories.
Conclusion:
This is a vast topic that is on the front end of scientific research in the last two decades. I tried to give a summary in this article before we go into some specific scenarios in correlated articles.
These are just some examples of epigenetic changes and their effects on cancer risk. Many more factors can cause epigenetic changes, such as smoking, exercise, stress, drugs, pollution, or trauma.
The bottom line is that your genes are not fixed. You can change them with your choices and you need to choose a diet rich in antioxidants, and fiber, avoiding bioaccumulation of mutagens and toxins in a food chain. You need to avoid over-caloric and dense nutrient-deprived diet types and incorporate caloric restriction with intermittent fasting and avoid over-expressing your endocrine system with excessive protein intake. There is a high level of correlation between overall cancer risk and chronically elevated IGF-1 levels (due to a high-quality protein-dominated SAD diet).

So make smart choices that protect your gene expression and reduce your cancer risk. My advice is not to wait for fifty more years before recommendations change, as we did with smoking. You have the power to change not just your genes but the genes of your children as well.
- Classical genetics could not explain everything, leading to the creation of molecular biology.
- Epigenetics challenged assumptions and dogmas of genetics and of molecular biology.
- Epigenetics exposes mechanisms that regulate gene expression without changing of DNA sequence.
- It is not genes that matter, but the way they express themselves.
- Chemical modifications on DNA or histones can affect how genes are accessed and used.
- Modifications can be added or removed by enzymes based on various factors.
- Changes can be passed down to daughter cells or even to children during reproduction.
- Epigenetics can influence traits not encoded by DNA independently, such as behavior, disease susceptibility, and aging.
- Gene expression refers to how often or when proteins are created from the instructions within genes.
- Transcription can be on or off depending on different factors, allowing the body to adapt to different situations.
- Cancer involves thousands of mutations in the genome and epigenome.
- Genetic predisposition is just one aspect of cancer.
- Gene expression, influenced by diet and environment, can turn on genetic predisposition for cancer.
- Epigenetic changes can affect the risk of developing cancer and responding to treatment.
- The high incidence of cancer in Western societies with a standard American diet is due to gene expression rather than bad genes.
- DNA methylation blocks gene access, turning genes off and deactivating the immune system.
- Methylation can increase cancer risk and make cancer more aggressive.
- Histone modification can affect genes that regulate the cell cycle, apoptosis, or angiogenesis.
- Mutations in various genes increase cancer risk, but additional regulation through methylation, histone modification, or noncoding RNA can further increase risk or make cancer harder to treat.
- Antioxidants can prevent or reverse DNA methylation caused by free radicals or toxins.
- Antioxidants can modulate histone modifications by influencing enzymes and changing chromatin structure.
- Antioxidants can regulate noncoding RNA, altering gene regulation.
- Antioxidants can prevent or repair DNA damage and restore normal gene expression, slowing down cancer development.
- Folate provides methyl groups for DNA methylation.
- Folate deficiency can impair DNA methylation and cause abnormal gene expression.
- Folate deficiency can impair cognitive function and increase the risk of dementia.
- Folic acid is not the same as folate.
- Calorie restriction slows down aging and modulates inflammation, oxidative stress, metabolism, and autophagy pathways.
- Calorie restriction increases the expression of genes that protect against cellular damage and death.
- Calorie restriction was a normal part of our evolution due to scarcity.
- Our body responds to restriction by repairing itself and slowing down metabolism.
- A whole food plant-based diet naturally induces calorie restriction while still meeting nutritional needs.
- Standard American Diet (SAD) leads to calorie excess and increased disease risk due to oil, sugar, and highly palatable foods.
- Fiber affects the gut microbiota by stimulating probiotic bacteria and downregulating numbers of non-probiotic meat-eating bacteria.
- Metabolites produced by the gut microbiota can affect organs and tissues and fiber can affect the type and amount of these metabolites.
- Fiber can modulate epigenetic regulation and alter gene expression involved in inflammation, immunity, and metabolism.
- Fiber can protect against obesity and diabetes by modulating gene expression involved in glucose homeostasis, lipid metabolism, and energy expenditure.
- Fiber can improve immune function by modulating gene expression involved in inflammation, immunity, and barrier function and by downregulating the number of non-probiotic bacteria.
- Fiber can stimulate the production of antibodies and cytokines to fight off germs.
- More fiber is usually better.
- Too much fiber can cause bloating, gas, and increased bowel movements.
- There are many factors that can cause epigenetic changes, such as smoking, exercise, stress, drugs, pollution, or trauma.
- Your genes are not fixed and can be changed by your choices.
- A diet rich in antioxidants and fiber is recommended while avoiding the bioaccumulation of mutagens and toxins in a food chain.
- Over-caloric and nutrient-deprived diets should be avoided, and caloric restriction with intermittent fasting is recommended.
- Excessive protein intake can overexpress the IGF1 and increase cancer risk.
FAQ
References:
- Peixoto, P., Cartron, P. F., Serandour, A. A., & Hervouet, E. (2020). From 1957 to Nowadays: A Brief History of Epigenetics. International journal of molecular sciences, 21(20), 7571. https://doi.org/10.3390/ijms21207571
- What is Epigenetics? | CDC. (2022, August 15). Centers for Disease Control and Prevention. https://www.cdc.gov/genomics/disease/epigenetics.htm
- LaPelusa, A., & Kaushik, R. (2022). Physiology, Proteins. In StatPearls. StatPearls Publishing. [PubMed]
- Blackwell, T. K., & Walker, A. K. (2006). Transcription mechanisms. WormBook : the online review of C. elegans biology, 1–16. https://doi.org/10.1895/wormbook.1.121.1
- Hamilton J. P. (2011). Epigenetics: principles and practice. Digestive diseases (Basel, Switzerland), 29(2), 130–135. https://doi.org/10.1159/000323874
- Fanucchi, S., Domínguez-Andrés, J., Joosten, L. A. B., Netea, M. G., & Mhlanga, M. M. (2021). The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity, 54(1), 32–43. https://doi.org/10.1016/j.immuni.2020.10.011
- Surace, A. E. A., & Hedrich, C. M. (2019). The Role of Epigenetics in Autoimmune/Inflammatory Disease. Frontiers in immunology, 10, 1525. https://doi.org/10.3389/fimmu.2019.01525
- Denhardt, D. T. (2018). Effect of stress on human biology: Epigenetics, adaptation, inheritance, and social significance. Journal of Cellular Physiology, 233(3), 1975–1984. https://doi.org/10.1002/jcp.25837
- de Magalhães-Barbosa, M. C., Prata-Barbosa, A., & da Cunha, A. J. L. A. (2022). Toxic stress, epigenetics and child development. Jornal de pediatria, 98 Suppl 1(Suppl 1), S13–S18. https://doi.org/10.1016/j.jped.2021.09.007
- Cavalli, G., & Heard, E. (2019). Advances in epigenetics link genetics to the environment and disease. Nature, 571(7766), 489–499. https://doi.org/10.1038/s41586-019-1411-0
- Brena, R. M., & Costello, J. F. (2007). Genome-epigenome interactions in cancer. Human molecular genetics, 16 Spec No 1, R96–R105. https://doi.org/10.1093/hmg/ddm073
- Shen, H., & Laird, P. W. (2013). Interplay between the cancer genome and epigenome. Cell, 153(1), 38–55. https://doi.org/10.1016/j.cell.2013.03.008
- Hullar, M. A., & Fu, B. C. (2014). Diet, the gut microbiome, and epigenetics. Cancer journal (Sudbury, Mass.), 20(3), 170–175. https://doi.org/10.1097/PPO.0000000000000053
- Abdul, Q. A., Yu, B. P., Chung, H. Y., Jung, H. A., & Choi, J. S. (2017). Epigenetic modifications of gene expression by lifestyle and environment. Archives of pharmacal research, 40(11), 1219–1237. https://doi.org/10.1007/s12272-017-0973-3
- Catteau, A., & Morris, J. S. (2002). BRCA1 methylation: a significant role in tumour development? Seminars in Cancer Biology, 12(5), 359–371. https://doi.org/10.1016/s1044-579x(02)00056-1
- Prajzendanc, K., Domagała, P., Hybiak, J., Ryś, J., Huzarski, T., Szwiec, M., Tomiczek-Szwiec, J., Redelbach, W., Sejda, A., Gronwald, J., Kluz, T., Wiśniowski, R., Cybulski, C., Łukomska, A., Białkowska, K., Sukiennicki, G., Kulczycka, K., Narod, S. A., Wojdacz, T. K., Lubiński, J., … Jakubowska, A. (2020). BRCA1 promoter methylation in peripheral blood is associated with the risk of triple-negative breast cancer. International journal of cancer, 146(5), 1293–1298. https://doi.org/10.1002/ijc.32655
- Yue, X., Zhao, Y., Xu, Y., Zheng, M., Feng, Z., & Hu, W. (2017). Mutant p53 in Cancer: Accumulation, Gain-of-Function, and Therapy. Journal of molecular biology, 429(11), 1595–1606. https://doi.org/10.1016/j.jmb.2017.03.030
- Saliani, M., Jalal, R., & Javadmanesh, A. (2022). Differential expression analysis of genes and long non-coding RNAs associated with KRAS mutation in colorectal cancer cells. Scientific reports, 12(1), 7965. https://doi.org/10.1038/s41598-022-11697-5
- Beetch, M., Harandi-Zadeh, S., Shen, K., Lubecka, K., Kitts, D. D., O’Hagan, H. M., & Stefanska, B. (2020). Dietary antioxidants remodel DNA methylation patterns in chronic disease. British journal of pharmacology, 177(6), 1382–1408. https://doi.org/10.1111/bph.14888
- Crider, K. S., Yang, T. P., Berry, R. J., & Bailey, L. B. (2012). Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Advances in nutrition (Bethesda, Md.), 3(1), 21–38. https://doi.org/10.3945/an.111.000992
- Gensous, N., Franceschi, C., Santoro, A., Milazzo, M., Garagnani, P., & Bacalini, M. G. (2019). The Impact of Caloric Restriction on the Epigenetic Signatures of Aging. International journal of molecular sciences, 20(8), 2022. https://doi.org/10.3390/ijms20082022
- Choi, S. W., & Friso, S. (2010). Epigenetics: A New Bridge between Nutrition and Health. Advances in nutrition (Bethesda, Md.), 1(1), 8–16. https://doi.org/10.3945/an.110.1004
- Borzabadi, S., Oryan, S., Eidi, A., Aghadavod, E., Daneshvar Kakhaki, R., Tamtaji, O. R., Taghizadeh, M., & Asemi, Z. (2018). The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin and Lipid in Patients with Parkinson’s Disease: A Randomized, Double-blind, PlaceboControlled Trial. Archives of Iranian medicine, 21(7), 289–295. [PubMed]
- Ye, J., Wu, W., Li, Y., & Li, L. (2017). Influences of the Gut Microbiota on DNA Methylation and Histone Modification. Digestive diseases and sciences, 62(5), 1155–1164. https://doi.org/10.1007/s10620-017-4538-6
- Bhattacharjee, S., & Dashwood, R. H. (2020). Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals. Antioxidants (Basel, Switzerland), 9(9), 865. https://doi.org/10.3390/antiox9090865
- Griñán-Ferré, Christian, et al. “Dietary Antioxidants, Epigenetics, and Brain Aging: A Focus on Resveratrol.” Oxidative Stress and Dietary Antioxidants in Neurological Diseases, edited by Colin R. Martin and Victor R. Preedy, Academic Press, 2020, pp. 343-57 https://doi.org/10.1016/B978-0-12-817780-8.00022-0
- Chhabria, S. V., Akbarsha, M. A., Li, A. P., Kharkar, P. S., & Desai, K. B. (2015). In situ allicin generation using targeted alliinase delivery for inhibition of MIA PaCa-2 cells via epigenetic changes, oxidative stress and cyclin-dependent kinase inhibitor (CDKI) expression. Apoptosis : an international journal on programmed cell death, 20(10), 1388–1409. https://doi.org/10.1007/s10495-015-1159-4
- Crider, K. S., Yang, T. P., Berry, R. J., & Bailey, L. B. (2012). Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Advances in nutrition (Bethesda, Md.), 3(1), 21–38. https://doi.org/10.3945/an.111.000992
- Saitsu, H. (2017). Folate receptors and neural tube closure. Congenital Anomalies, 57(5), 130–133. https://doi.org/10.1111/cga.12218
- Abraham, K. J., Ostrowski, L. A., & Mekhail, K. (2017). Non-Coding RNA Molecules Connect Calorie Restriction and Lifespan. Journal of molecular biology, 429(21), 3196–3214. https://doi.org/10.1016/j.jmb.2016.08.020
- Patel, S. A., Velingkaar, N., Makwana, K., Chaudhari, A., & Kondratov, R. (2016). Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Scientific reports, 6, 25970. https://doi.org/10.1038/srep25970
- Gabandé-Rodríguez, E., Gómez de Las Heras, M. M., & Mittelbrunn, M. (2019). Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells, 9(1), 82. https://doi.org/10.3390/cells9010082
- Bagherniya, M., Butler, A. E., Barreto, G. E., & Sahebkar, A. (2018). The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing research reviews, 47, 183–197. https://doi.org/10.1016/j.arr.2018.08.004
- Komatsu, T., Park, S., Hayashi, H., Mori, R., Yamaza, H., & Shimokawa, I. (2019). Mechanisms of Calorie Restriction: A Review of Genes Required for the Life-Extending and Tumor-Inhibiting Effects of Calorie Restriction. Nutrients, 11(12), 3068. https://doi.org/10.3390/nu11123068
- Greger M. (2020). A Whole Food Plant-Based Diet Is Effective for Weight Loss: The Evidence. American journal of lifestyle medicine, 14(5), 500–510. https://doi.org/10.1177/1559827620912400
- Makki, K., Deehan, E. C., Walter, J., & Bäckhed, F. (2018). The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell host & microbe, 23(6), 705–715. https://doi.org/10.1016/j.chom.2018.05.012
Related Posts
Do you have any questions about nutrition and health?
I would love to hear from you and answer them in my next post. I appreciate your input and opinion and I look forward to hearing from you soon. I also invite you to follow us on Facebook, Instagram, and Pinterest for more diet, nutrition, and health content. You can leave a comment there and connect with other health enthusiasts, share your tips and experiences, and get support and encouragement from our team and community.
I hope that this post was informative and enjoyable for you and that you are prepared to apply the insights you learned. If you found this post helpful, please share it with your friends and family who might also benefit from it. You never know who might need some guidance and support on their health journey.
– You Might Also Like –

Learn About Nutrition
Milos Pokimica is a doctor of natural medicine, clinical nutritionist, medical health and nutrition writer, and nutritional science advisor. Author of the book series Go Vegan? Review of Science, he also operates the natural health website GoVeganWay.com
Medical Disclaimer
GoVeganWay.com brings you reviews of the latest nutrition and health-related research. The information provided represents the personal opinion of the author and is not intended nor implied to be a substitute for professional medical advice, diagnosis, or treatment. The information provided is for informational purposes only and is not intended to serve as a substitute for the consultation, diagnosis, and/or medical treatment of a qualified physician or healthcare provider.NEVER DISREGARD PROFESSIONAL MEDICAL ADVICE OR DELAY SEEKING MEDICAL TREATMENT BECAUSE OF SOMETHING YOU HAVE READ ON OR ACCESSED THROUGH GoVeganWay.com
NEVER APPLY ANY LIFESTYLE CHANGES OR ANY CHANGES AT ALL AS A CONSEQUENCE OF SOMETHING YOU HAVE READ IN GoVeganWay.com BEFORE CONSULTING LICENCED MEDICAL PRACTITIONER.
In the event of a medical emergency, call a doctor or 911 immediately. GoVeganWay.com does not recommend or endorse any specific groups, organizations, tests, physicians, products, procedures, opinions, or other information that may be mentioned inside.
Editor Picks –
Milos Pokimica is a health and nutrition writer and nutritional science advisor. Author of the book series Go Vegan? Review of Science, he also operates the natural health website GoVeganWay.com
Latest Articles –
Top Health News — ScienceDaily
- Study finds vegetarians over 80 less likely to reach 100on February 26, 2026
Avoiding meat might slightly lower the odds of reaching 100 — but only for frail, underweight seniors. In very old age, staying strong and maintaining muscle matters more than long-term disease prevention. Older adults who included fish, eggs, or dairy were just as likely to become centenarians as meat eaters, suggesting that key nutrients may make the difference. The takeaway: nutrition needs change dramatically with age.
- Shingles vaccine may slow biological aging and reduce inflammationon February 26, 2026
A shingles shot might do more than prevent a painful rash — it could actually help slow down the aging process. In a large national study of more than 3,800 Americans age 70 and older, those who received the shingles vaccine showed slower biological aging compared to those who didn’t. Researchers found lower levels of chronic inflammation and slower changes in gene activity linked to aging, suggesting the vaccine may calm the body’s “inflammaging” — the low-grade inflammation tied […]
- Massive review suggests exercise may do little for osteoarthritis painon February 26, 2026
A sweeping new analysis of the evidence suggests that exercise therapy — long promoted as a first-line treatment for osteoarthritis — may offer only small and short-lived relief, and in some cases might be no better than doing nothing at all. After reviewing dozens of clinical trials involving more than 13,000 participants, researchers found that benefits for knee osteoarthritis pain were minimal and tended to shrink in larger or longer-term studies.
- Just two days of oatmeal cut bad cholesterol by 10%on February 25, 2026
Eating nothing but oatmeal for just two days might sound extreme, but it delivered a striking payoff in a new clinical trial. People with metabolic syndrome who followed a short, calorie-reduced oat-based plan saw their harmful LDL cholesterol drop by 10%, along with modest weight loss and lower blood pressure. Even more surprising, the cholesterol benefits were still visible six weeks later.
- New drug target discovered for devastating “brain on fire” diseaseon February 25, 2026
Scientists have zeroed in on a critical weak spot behind a rare but devastating brain autoimmune disorder often known as “Brain on Fire.” The disease strikes when the immune system attacks NMDA receptors—key molecules involved in memory and thinking—leading to psychiatric symptoms, seizures, and even death.
- Scientists discover hidden sugar layer behind psoriasison February 25, 2026
A gel-like sugar coating on immune cells has been found to play a starring role in psoriasis. Researchers discovered that immune cells shed this outer layer to help them exit the bloodstream and enter inflamed skin. This challenges the long-held idea that only blood vessel walls changed during this process. The finding could help guide new therapies aimed at controlling harmful inflammation.
- New brain stimulation approach could treat depression in just 5 dayson February 25, 2026
A weeklong, high-intensity version of TMS may work nearly as well as the standard six-week treatment for depression. In a UCLA study, patients who received five sessions a day for five days experienced meaningful symptom relief comparable to those on the traditional schedule. Some who didn’t improve immediately showed strong gains weeks later. The findings hint at a faster, more accessible path to recovery.
PubMed, #vegan-diet –
- Veganism: an extended theory of planned behavior framework incorporating ethical, environmental, and sociodemographic determinantson February 20, 2026
CONCLUSION: This study broadens the TPB by integrating ethical, normative, and psychosocial dimensions that explain vegan intentions beyond traditional predictors. Findings underscore the importance of moral identity, perceived social expectations, and contextual factors in shaping sustainable dietary behaviors.
- Association Between Diet and Metabolome in Childhood and Adolescence: A Systematic Reviewon February 11, 2026
CONCLUSION: This review identifies several metabolites consistently associated with specific dietary components across different studies in children and adolescents. These findings support the potential of metabolomics for validating dietary biomarkers and improving the accuracy of dietary assessment in pediatric populations. Although metabolomic markers reflect actual dietary intake, their implications for health outcomes remain to be explored.
- Growth Trajectories in Infants From Families With Plant-Based or Omnivorous Dietary Patternson February 5, 2026
CONCLUSIONS AND RELEVANCE: In this cohort study, infants from vegan households had growth patterns similar to those from omnivorous households, with a higher odds of early underweight that decreased by age 24 months. In the context of developed countries, these findings seem reassuring. Further research should examine vegan diet quality and the impact of nutritional counseling during pregnancy and infancy in supporting optimal infant development.
- Influences of vegan status on protein intake, lean body mass, and strength in lightly active, young women: A cross-sectional studyon February 5, 2026
CONCLUSION: These data suggest that functional indicators of body protein status may be adversely impacted by long-term adherence to vegan diets in young adult women.
- Iodineon January 1, 2006
Iodine is an essential trace nutrient for all infants that is a normal component of breastmilk. Infant requirements are estimated to be 15 mcg/kg daily in full-term infants and 30 mcg/kg daily in preterm infants.[1] Breastmilk iodine concentration correlates well with maternal urinary iodine concentration and may be a useful index of iodine sufficiency in infants under 2 years of age, but there is no clear agreement on a value that indicates iodine sufficiency, and may not correlate with […]
Random Posts –
Featured Posts –
Latest from PubMed, #plant-based diet –
- Impact of nutrition on long COVIDby Subramanian Thangaleela on February 25, 2026
Long COVID is characterized by a group of persistent symptoms following the acute SARS-COV2 infection, which presented a multifaceted challenge to the healthcare systems all over the globe. The long COVID symptoms span various organ systems including the respiratory, cardiovascular, gastrointestinal, and neurological manifestations. Mitochondrial dysfunction and immune dysregulation play crucial roles in the long COVID pathophysiology. Recently nutritional intervention gained much attention […]
- Lifestyles, leisure activities, subjective well-being, and long-term survival after age 80 in China: a population-based cohort studyby Xinye Zou on February 25, 2026
CONCLUSIONS: The study findings have demonstrated that adopting a healthy lifestyle, engaging in leisure activities, and fostering subjective well-being are associated with longer life expectancy among the oldest old in China.
- Differences in Protein Quantity and Quality Across a Spectrum of Plant-Based Meals: Analysis of a Large National Dietary Surveyby Sophie L van Oppenraaij on February 24, 2026
CONCLUSIONS: This study shows that only a small proportion of Dutch adults met both protein-related recommendations and sustainability goals, due to lower protein quantity and quality in more plant-based diets. This study emphasizes the need for professional guidance, especially in individuals with higher protein requirements, to facilitate a successful transition to a more plant-based diet.
- Dietary animal fat disrupts gut microbiota and aggravates Scl-cGVHD after allogeneic hematopoietic stem cell transferby Danielle D Millick on February 24, 2026
Allogeneic hematopoietic stem cell transplant (allo-HSCT) is an effective treatment for high-risk or relapsed acute leukemia. However, the frequent occurrence of graft-versus-host disease (GVHD) poses significant complications. Modifiable factors such as the gut microbiome and dietary regimen have the potential to influence the frequency and severity of GVHD. Previous studies in mouse models have shown a direct link between obesity and increased severity of GVHD; however, analysis of human […]
- Association between the adherence to different dietary patterns and sperm chromatin integrity in healthy menby Marc Llavanera on February 24, 2026
CONCLUSION: To the best of our knowledge, this study is the first to epidemiologically investigate the relationship between dietary patterns and sperm chromatin integrity, highlighting that adherence to unhealthy plant-based diets may lead to sperm chromatin abnormalities. These findings underscore the potential effect of specific dietary patterns on molecular sperm quality parameters and support further research into dietary strategies for optimizing sperm integrity and improving male…
- Evaluation of biochemical, histopathological, hematological, and genotoxic effects of some indigenous weed plant extracts in albino rats toward a natural and safe alternative to synthetic insecticidesby Muhammad Asif Zahoor on February 23, 2026
CONCLUSION: These findings suggest that these weed plants have the potential to be used as biopesticides for future integrated pest management (IPM) programs.